Naloxone is a medication that temporarily stops heroin and other opioid substances from reaching the brain. When used promptly, it can help stop a person from dying from an opioid overdose. In recent years, public health officials and researchers have sought to increase the availability of naloxone and make the drug easier to administer in real-world circumstances. In 2014, the U.S. Food and Drug Administration gave priority status to the development of a new, inhaled form of naloxone that may significantly boost the medication’s effectiveness in fatal overdose prevention.
Opioid Overdose
Opioid overdose is the consequence of excessive, short-term opioid intoxication. It occurs because, in addition to their ability to produce powerful feelings of euphoria, opioid drugs like heroin and opioid medications like oxycodone substantially slow down the baseline rate of activity inside the brain and spinal cord (i.e., central nervous system). When your central nervous system activity falls below a certain point, your body can fail to perform such essential tasks as keeping your heart beating and drawing in oxygen through your lungs. Unless corrected rapidly, disruption of these critical functions can quickly kill you. However, not all people experience fatal opioid overdoses; some individuals receive help in time, while others don’t take enough of an opioid substance to fatally compromise their central nervous system function. The Centers for Disease Control and Prevention track the number of people in the U.S. who experience fatal overdoses related to the use of prescription opioid medications. In 2011 (the last year with fully reported figures), 16,917 such overdose deaths occurred. Significant numbers of people who start out abusing prescription opioids eventually become IV heroin users and thereby seriously heighten their overdose risks. The rate of overdose has increased fairly steadily in America since the beginning of the 21st century. Population groups most likely to experience a fatal or nonfatal overdose include men, European Americans and people in their mid- to late 40s.
Naloxone
In order to produce their effects, opioid substances must reach your central nervous system through sites called opioid receptors, which are found on cells in your brain, spinal cord, intestinal tract and certain other body areas. Naloxone essentially blocks access to these receptors for a short period of time, and thereby counters opioid intoxication. Currently, all naloxone products come in an injectable form. Prior to 2014, safe use of the medication required medical training and knowledge of proper injection practices. However, in April 2014, the FDA approved an automatically injected form of naloxone branded as Evzio. Like some defibrillators, this product relies on recorded, spoken instructions to relay information on its proper use. Evzio was approved, in part, in response to the recent rise in opioid abuse and fatal incidents of opioid overdose. It was also approved in response to a widely perceived need for a form of naloxone that does not rely on a doctor or other trained medical personnel for safe use. Most states in the U.S. don’t allow people outside of the medical profession to administer naloxone in its traditional injected form.
New Form of Naloxone
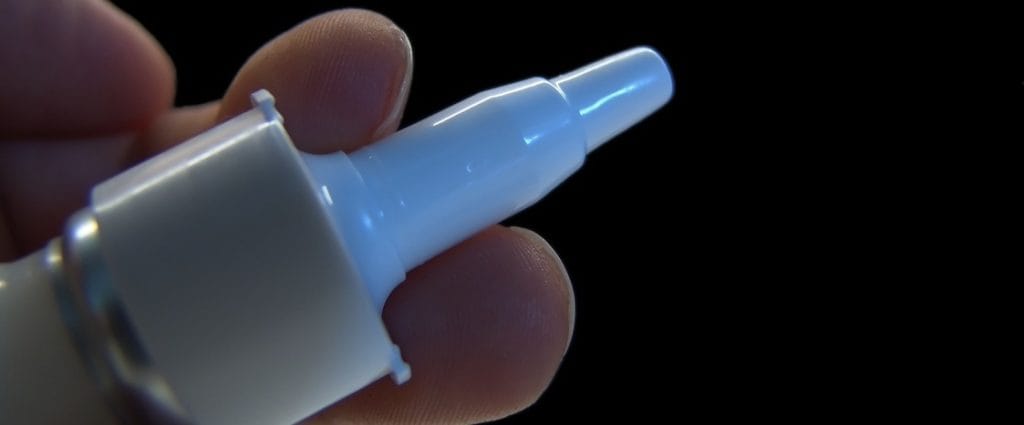
In August 2014, researchers from the University of Kentucky announced progress on the development of an inhaled naloxone product. This product introduces a single, predictable dose of the medication to the interior of the nostrils, where it gets absorbed through the mucous membranes that line the nasal cavity. Even if a person in the midst of an opioid overdose is no longer breathing, this method of administration ensures that the medication reaches the bloodstream and has its desired blocking effect on the body’s opioid receptors. The creator of this naloxone product specifically cited a desire to increase safe access to the medication as a motivation for his work. Perhaps not coincidentally, Kentucky has experienced a particularly sharp rise in fatal opioid overdoses in recent years. The FDA has fast-tracked the approval process for the new nasal naloxone product. Fast-tracked medications go through an accelerated development phase, as well as an accelerated phase of official review before final approval. As a rule, all medications that receive this type of priority attention address health concerns that are severe and/or potentially fatal and currently lack sufficient options for timely, effective treatment. Evzio also went through a fast-tracked process before its approval. The new, nasal form of naloxone is now going through its final period of testing in humans.